Journal of
eISSN: 2373-4396


Review Article Volume 16 Issue 2
Department of Internal Medicine, The University of Campania Luigi Vanvitelli, Italy
Correspondence: Federico Cacciapuoti, Department of Internal Medicine “L. Vanvitelli” Campania University, Naples, Italy
Received: March 20, 2023 | Published: April 12, 2023
Citation: Cacciapuoti F. Hyperhomocysteinemia acts via DNA-hypomethylation to induce atherosclerosis. J Cardiol Curr Res. 2023;16(2):38-41. DOI: 10.15406/jccr.2023.16.00575
Hyperhomocysteinemia (HHcy) is an independent risk factor for atherosclerosis responsible for chronic and acute cardiovascular events, such as myocardial infarction, stroke, and peripheral vascular disease. The aim of this review is to evaluate the mechanisms through which increased homocysteine (Hcy) levels cause atherosclerotic events. It is knonw that the amino-acid Hcy, through the trans-methylation pathway, results in S-adenosyl-methionine (SAM). In turn, SAM transfers a methyl group (-CH3) to some substrates, such as DNA, turning in S-Adenosyl-Homocysteine (SAH). But, this compound is able to inhibit DNA methyltransferase (DNT), that is the enzyme responsible for DNA methylation. The consequent DNA hypomethylation favors the Cyclin A inhibition, responsible for the atherosclerotic findings. Thus, DNA hypomethylation is a risk factor for atherosclerosis rather than HHcy, that is a simple indicator of this complication. Concordantly, several reports and large trials indicate that folate (vit. B-9) and B-6-12 vitamins supplementation, even lowers HHcy levels, did not reduce the incidence of atherosclerosis. But, that can be antagonized by the product of Hcy-transsulfuration, as H2S. Conclusively, the contemporary administration of H2S + folic acid (that antagonizes HHcy) should reduce both high Hcy serum levels and cardiovascular acute events.
Keywords: hyperhomocysteinemia; S-adenosyl-methionine (SAM) S-adenosyl-homocysteine (SAH); DNA hypomethylation (DNT); atherosclerosis, folate; B-6-12 vitamins supplementation, H2S
CpG, cytosine phosphate guanine; C5, cytosine 5; RNA, ribo-nucleic acid; HHcy, hyper-hmocysteine; Hcy, homocysteine; Met, methionine; ATP, adenosine-tri phosphate; MAT, methionine adenosyl transferase; SAM, S-adenosyl-methionine; SAH, S-adenosyl-homocysteine; SAHH, S-adenosyl homocysteine hydrolase; VSMCs, vascular smooth muscle cells; ECs, endothelial cells; PDGF, platelet derived growth factor; H2S, hydrogen sulfide
DNA methylation is a biological process induced by the addition of a methyl group (-CH3) to DNA. The biochemical process happens in CpG (Cytosine-phosphate-Guanine) island. It involves the addition of CH3 to the C5 position of cytosine (5 methyl-cytosine-5mC-).1 The process essential for the body’s development. In fact, DNA methylation controls gene expression, DNA conformation, and DNA stability.2 On the contrary, DNA hypomethylation refers to the loss of CH3 in the methyl-cytosine nucleotide. This process causes changes in the genome functions (phenotypic) without changes of the DNA sequence (genotypic).3,4
DNA hypomethylation is a part of “Epigenomics”, term coined by Conrad Waddington in 1942. It is an event reversible that involves four systems (DNA-hypomethylation, histone modifications, chromatin architecture and non-coding RNA)5,6 Here, we debated about global DNA hypomethylation only and its involvement in the pathogenesis of atherosclerosis. DNA-global hypomethylation refers to the total level of 5mC content, that may induce genome instability and deregulate gene transcription thereby contributing to the development of various human diseases, including tumors and vascular diseases (atherosclerosis).7 A most important mechanism causing DNA hypomethylation depends on the increased serum levels of homocysteine (HHcy).8
Homocysteine
Homocysteine (Hcy) is sulfhydryl amino acid derived from the essential amino acid Methionine (Met). Met is contained in some foods, as fish, eggs, grains, and dairy diet products.9 Normally, fasting Hcy concentration in plasma changes from 5 to 15µmol/L. A value>15µmol/L is defined as HHcy. That is classified as mild (15 to 30µmol/L), moderate (30 to 100µmol/L), and severe (>10µmol/L).10,11
Hcy (previously obtained from Met) is further recycled in Met. The compound receives an adenosine group from ATP, through a reaction catalyzed by the enzyme adenosyl-Methionine-synthetase (MAT), and is converted in S-Adenosyl-Methionine (SAM), via trans-methylation pathway. After the transfer of the methyl group (-CH3) to an acceptor molecule, SAM is converted in S-Adenosyl-Homocysteine (SAH). Among the substrates receiving a methyl group (CH3) are comprised proteins, amino acids phospholipids, neurotransmitters DNA, RNA, etc. These trans-methylation reactions happen through the enzymes called methyltransferases (MTs).12 But, these enzymes are inhibited by SAH and SAM/SAH ratio can be considered as a specific index of methylation reactions and represents a sensitive biomarker of the transmethylation reactions.13,14 The SAM/SAH index decreases when SAM concentration lowers and SAH value rises and vice versa. Subsequently, SAH is turned into Hcy + Adenosine, by the enzyme SAH-Hydrolase (SAHH) (Figure1). This reaction is reversible and therefore, in presence of increased Hcy concentration, it favors the SAH-synthesis rather than SAH- hydrolysis. But, the increased SAH value inhibits MTs, inducing the hypomethylation of the substrates,15,16 as illustrated in figure.17 On account of these considerations, it appears evident that SAH, rather than HHcy is a true risk factor for the substrates’ hypomethylation. That happens for s DNA hypomethylation.18–20 It is also evident that the increased plasma concentration (HHcy) has a positive correlation with SAH levels and a negative correlation with DNA methylation. In detail and with reference to our specific topic, HHcy (favoring the SAH accumulation) inhibits the activity of DNA methyltransferase (DNMTs), causing DNA hypomethylation.21,22 In turn, this process originates is a cause of tha pathogenetic mechanism of the atherosclerosis.23
Atherosclerosis
HHcy is an independent risk factor for atherosclerosis. This is a complex disease characterized by the accumulation of vascular lipids, immune system activation, oxidative stress, endothelial cell (ECs) activation, vascular (arterial) smooth muscle cell (VSMC) proliferation, foam cell formation and macrophages’ activation. These changes are strongly favored by DNA hypomethylation, an important mechanism which changes gene expression.23,24. The underlying causes of these changes were before largely unknown.25. But, in the year 2007, Jamaluddin and coworkers firstly proposed that the apparent connection between HHcy and these atherosclerotic stigmatas26 realty is due to the DNA hypomethylation coming from HHcy.27,28 In addition, another study performed by Wang and coll. affirms that the direct cause of atherosclerosis is the ECs dysfunction derived by the inhibition of Cyclin A transcription, via DNA hypomethylation.29 Cyclin A is a member of the Cyclin family, a group of proteins that regulates the progression through the cell cycle.30 In detail, Cyclin A works as a regulator of the cell cycle progression in late phase G1 (mitosis) and in S phase (meiosis).31 The suppression of Cyclin A is responsible for ECs inhibition (ECs dysfunction) of the intima layer.32 The vascular media, mainly composed of VSMCs, is implicated in maintaining the structural integrity and the physiological function of the arterial vessels. DNA hypomethylation intervenes in the VSMCs proliferation via multiple mechanisms, such as the aberrant migration of platelet-derived growth factor (PDGF),33-35 histone modifications 36 non-coding RNAs 37 and others.38 In particular, PDGF (a dimeric glycoprotein belonging to the family of vascular endothelial growth factors), acts to regulate cell growth and proliferation. Similary, DNA hypomethylation (but not HHcy) directly acts in the formation of human atherosclerotic plaques.39 Both ECs dysfunction and VSMC proliferation are indirectly dependent of HHcy via DNA hypomethylation, as illustrated in Figure2& 3.
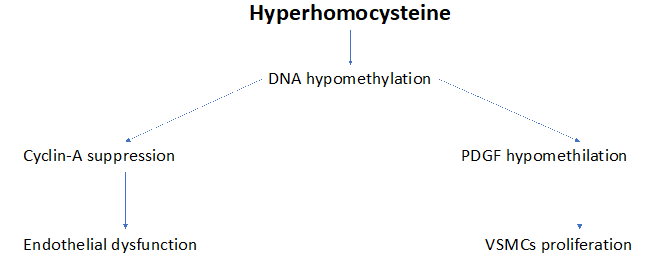
Figure 3 Diverse pathways through DNA hypomethylation induces endothelial dysfunction and VSMCs proliferation.
Treatment with B vitamins
It is known that folate administration reduces HHcy but is unable to prevent the atherosclerotic complications due to HHcy. Concerning this, a previous study demonstrated that folic acid (5mg) supplementation resulted in a reduction of Hcy concentration of 25%. An additional reduction of 7% was obtained by adding vitamin B12 to folic acid.40 But, the prevention of atherosclerotic cardiovascular events wasn’t evaluated in this report. Jung et al. recently confirmed that folic acid supplementation in these patients, even if reduces HHcy levels, has no effects on global DNA methylation.41 Nevertheless, in an experimental study, Cui et al. found that the folic acid supplementation wasn’t only able to reduce elevated Hcy concentration, but is useful to inhibit the raised SAM concentration and to lower the SAH synthesis, raising the methylation index (SAM/SAH ratio). The protective mechanism of the folic acid on the vascular walls seems to be dependent of to improve the DNA methyltransferase activity.42 In addition, a recent study of Kaye et al. concluded that folic acid supplementation may likely reduce vascular disease.43 Thus, the relationship between the folic acid supplementation and the vascular injury in humans suffering from HHcy is mixed.44-47 A dual response to folic acid supplementation was obtained after the fortification with folic acid, depending on the duration time of the food’s fortification. Yang et al. found a decrease of ischemic stroke in HHcy-patients resident in some Countries (US, Canada) where folic acid fortification of the cultivations was already carried out at time of patients-recruitment. These findings are consistent with the hypothesis that folic acid fortification contributes to the reduction of cardiovascular risk (stroke especially). Contrarily, stroke mortality did not change significantly in England and Wales in the same period (1998-2002), since the cultivations’ fortification weren’t realized yet at time of patients-recruitment.48 Concerning that, the WENBIT (Western Norway B Intervention Trial) study performed in Western Norway, a region non-fortified with folic acid, did not demonstrate a beneficial effect of B vitamins on total mortality or cardiovascular events.49 Another condition for obtain an improvement of cardiovascular events with folic acid supplementation without significant reduction of Hcy levels, was the coexistence of chronic kidney failure with HHcy, such as reported by Wrone et al.50 Concordantly, Righetti et al. also referred a positive effect on the incidence of cardiovascular accidents without a reduction of HHcy, in a little number of dialysis patients.51 In addition, Liem et al. reported an improvement of cardiovascular events without reduction of Hcy values, in HHcy-patients, when statins were added to folic acid.52 Furthermore, H2S, a bioproduct of Hcy metabolism, has been shown to antagonize Hcy’s effects and its vascular protective.53–55 Finally, the meta-analysis by Bazzano et al. reported no significant benefits by folic acid supplementation on the risk of cardiovascular disease and/or stroke. But these negative results were only recorded among the patients already suffered of these diseases (secondary prevention). Contrarily, a significant reduction of cardiovascular events was obtained in primary prevention.56
As it’s evident, multiple reasons may justify the conflicting results deriving from the folic acid supplementation to prevent the vascular atherosclerosis in patients suffering of HHcy., such as the different cardiovascular disease of the population enrolled (myocardial infarction, stroke, diabetes mellitus, hypertension, chronic kidney disease and other vascular diseases) must be considered.57 Another condition that may influence the results is the presence or not of acid acid-fortification of the cultivations at time of the patients-recruitment. The time-duration (from 2 to 7years) of vitamins B supplementation must be also considered. But, the most important condition influencing the improvement or not of cardiovascular events in individuals suffering from HHcy is the mode of cardiovascular prevention (primary or secondary). Concerning that, folic acid supplementation is unable to prevent the inflammation, the cells’ proliferation and the atherosclerotic lesions in HHcy-patients already suffered from one or more cardiovascular events.
Nevetheless, the supplementation only slows the initial displays of atherosclerosis.58 In other words, folic acid supplementation seems to reduce the progression of early-stages of atherosclerosis, but not the progression of markers of late-stages of atherosclerosis.59 But, H2S, product of Hcy transsulfuration, even if has no effect in to reduce the high Hcy values, is cardioprotective against fibrosis and hypertrophy and significantly reduces collagen deposition in the left ventricle of a mice-model. The compound has also been shown to shorten VSMCs proliferation, increase NO production and inhibit angiotensin converting enzyme of endothelial cells.60
Conclusively, even if HHcy is esteemed such as an independent risk-factor of early and massive atherosclerosis, multiple experiences indicate that HHcy is a simple marker rather than a true risk factor for atherosclerosis. Consequently, the reduction of HHcy-values through folic acid and/or vitamins B6-12 supplementation decreases the high values of Hcy, but does not prevent the atherosclerotic events. In fact, these are a consequence of reduced DNA methylation (DNA hypomethylation) rather than for reduction of Hcy “per se”. That explains the failure, giving folic acid, to reduce vascular acute events. But, this failure comes in secondary prevention (when these events are already happened). On the contrary, the administration of folic acid before the incidence of cardiovascular acute events can prevent or reduce these same (primary prevention). Likewise, the folic acid fortification of the grain cultivations can prevent some vascular events (stroke especially) only when the fortification was yet happened before the patients’ recruitment. It must be also undelined that the product of the Hcy transsulfuration, such as H2S are useful in to improve some cardiovascular disorders , even if they are unable to lower the high Hcy values. Therefore, a suitable treatment for both high Hcy levels (HHcy) and their cardiovascular disorders (atherosclerosis) should be folic acid + a donor of H2S (N-Acetyl-Cysteine-NAC).54 But, on purpose other experiences must be performed in future to contemporarily antagonize HHcy, their early atherosclerosis and the consequent cardiovascular acute events.
None.
The author declared no potential conflicts of interests.
The author received no financial support for the research and/or publication of this article.

©2023 Cacciapuoti. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.