Journal of
eISSN: 2469 - 2786


Research Article Volume 5 Issue 4
1Department of Applied Sciences, University of Technology, Iraq
2Department of Chemical Engineering, University of Technology, Iraq
Correspondence: Barbooti MM, Department of Applied Sciences, University of Technology, P.O. Box 35045, Baghdad, Iraq
Received: June 30, 2017 | Published: October 12, 2017
Citation: Barbooti MM, Ibrahim NK, Alwan AH. Design of experiments for the optimizationof biochemical treatment of tannery wastewater. J Bacteriol Mycol Open Access. 2017;5(4):328-333. DOI: 10.15406/jbmoa.2017.05.00141
The present work is concerned with the finding of the optimum conditions for biochemical wastewater treatment for a local tannery. The water samples were taken from outline areas (the wastewater of the chrome and vegetable tannery) in equal volumes and subjected to sedimentation, biological treatment, chemical and natural sedimentation treatment. The Box-Wilson method of experimental design was adopted to establish relationships between three operating variables that affect the treatment processes (temperature, aeration period and phosphate concentration) and the Biochemical Oxygen Demand (BOD5). The experimental data collected by this method were successfully fitted to a second order polynomial mathematical model. The most favorable operating conditions for the treatment were; a temperature, 32.5°C, 10hours aeration period and phosphate concentration of 16.8mg/L. On using the optimum conditions a mathematical model simulating the operation for the treatment was obtained.
Keywords: activated sludge, biological treatment, experimental design, optimization, tannery wastewater
Almost all tanneries are located on rivers to provide them with process water (30 to 80 m3 for 1 ton of processed raw skins)1 and for the disposal of their effluents. The effluent from this industry is mainly waterborne.2 The components of the effluent arise from purification of raw hides and skins before processing as well as from residual chemicals from the production processes. Hence, the tanning industry had been recognized as a major contributor to water pollution problems. Biodegradable organic matter consumes oxygen and nutrients in complex biochemical reactions until rendered inert.3 This exerts a biochemical oxygen demand as one of the fundamental parameters used to regulate the quality of the effluent.4
The contaminants in industrial wastewater are removed by physical, chemical and biological means.5,6 Facilities for handling wastewater are usually considered to have three major parts: collection, treatment and disposal.7 Pre-aeration is used to “freshen” the wastewater and to assist the removal of oil and grease.8 Secondary treatment processes commonly consist of biological processes. This means that living organisms which control the environment of the process are used to partially stabilize (oxidize) organic matter not previously removed by treatment processes and to convert it into a form which is easier to remove from the wastewater.9
Sedimentation or primary treatment makes the wastewater much clearer. Two clarifiers are used10 to provide detention time (3h) where, almost 60% of the suspended solids (SS) will either settle to the bottom or float to the surface and be removed. Removal of these solids will usually reduce the BOD5 of the waste approximately 35%.
The next step is the biological treatment which can typically be divided into aerobic and anaerobic. Anaerobic biological treatment is an oxygen-devoid process. Aerobic biological treatment is done in the presence of oxygen. It is applicable to wastewater containing bio-degradable organic constituents and some non-metallic inorganic constituents.11
The activated sludge process (ASP) is the currently used biological treatment process for wastewater in Al-Za’afaraniya tanning factory, southern Baghdad. The system consists of an equalization basin, a settling tank, an aeration basin, a clarifier, and a sludge line. The recirculation of the biomass, which is an integral part of the process, allow microorganisms to adapt changes in wastewater composition with relatively short acclimation time and also allow a greater degree of control over acclaimed bacterial population.12 For a proper control of the ASP, the growth of the micro-organisms should be controlled. Bacteria make up about 95% of the activated sludge biomass. These single celled organisms grow in the wastewater by consuming (eating) biodegradable materials such as proteins, carbohydrates, fats and many other compounds. Some important factors acting on growth and activity of bacteria in biochemical wastewater treatment are: food-to-microorganism ratio (F/M); use of oxygen;13 formation of Floc;14 mixing;15 pH;16 temperature [6] and the effect of nutrients.16
Biological oxygen demand (BOD) is a measure of the oxygen used by microorganisms to decompose this waste. A large quantity of organic waste in the water requires large amount of bacteria to decompose it. Thus, the demand for oxygen will be high (high level of BOD). As the waste is consumed or dispersed through the water, BOD levels will begin to decline.17
Nitrates and phosphates in a body of water can contribute to high BOD levels. Nitrates and phosphates are plant nutrients and can cause plant life and algae to grow quickly. When plants grow quickly, they also die quickly. This contributes to the organic waste in the water, which is then decomposed by bacteria. This results in a high BOD level.18 When BOD levels are high, dissolved oxygen (DO) levels decrease because the oxygen that is available in the water is being consumed by the bacteria. Since less dissolved oxygen is available in the water, fish and other aquatic organisms may not survive.19
According to a recent UNIDO report,20 the treatment of tannery effluents is by now a well established technology, and modular common effluent treatment plants servicing traditional tannery clusters or newly created leather industry zones is a widely accepted approach. However, two issues still pose serious challenges.
High total dissolved solids, TDS, unaffected by treatment. This problem is especially pronounced in developing countries where mixing tannery effluent with domestic sewage or its discharge into the sea is not feasible, and the raw hides and skins are still preserved by salting. Relocation of tanneries to the seaside is often not feasible, and desalination of treated effluent by reverse osmosis is very expensive. However, utilization or safe disposal of sludge, cost-effective solutions to both of these problems are still eagerly awaited.
The conventional treatment system marginally removes the organic pollutant where as it failed to remove total dissolved solids. The treated water it can be understand that the treated water can be reused for the process. The rejects obtained in the process is subjected to solar evaporation system or multiple evaporation system for further recovery of salt.21 The design of experimenst system provides a valuable approach to study effects of multiple variables on the performance of various processes. Studies on environmental issues benefited well from the system and many examples may be presented here. Delgado-Moreno et al.22 studied the extraction of triazines from soil after olive cake amendment. Experimental design was succesfully emplyed to evaluate the optimal conditions on the removal of oxytetracycline and heavy metals from water.23,24 The systems presents a systematic approach for the effects of simultaneous variables at several levels and allows the evaluation of the applicability of the proposed model.
The present work is an attempt to apply the design of experiments approach to shed more light on the pollution potential of a state tanning factory, South of Baghdad, Iraq. The BOD5 is taken as a parameter to indicate the optimum condition of biological treatment in ASP.
Materials
Phosphoric acid (70 wt%) was supplied by a local company. The chemicals used for the determination of BOD5 and phosphate were of analytical grade. An agar was prepared for the growth of bacteria, and consists of: beef extract, 3g; peptone, 5g; agar, 15g and distilled water to make a volume of one liter. The pH of the culture media was 7.0.
Malt extract agar was used to grow fungi. it consists of: malt extract, 20g ; peptone, 5g, agar, 15g and distilled water to make a volume of one liter. Culture media: pH = 3.5-4.0.
Laboratory treatment units
Figure 1 shows a schematic diagram of the experimental unit used for the biochemical wastewater treatment. The unit consists of:
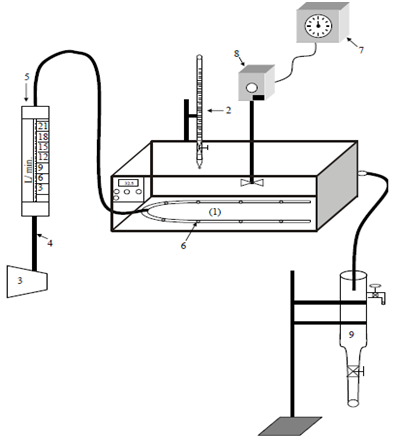
Figure 1 Schematic diagram of the experimental system: 1: Water bath; 2: Burette; 3: Compressor; 4: Pipes; 5: Rotameter; 6: Air sparger; 7: Voltage Controller Box; 8: Stirrer and 9: Clarifier.
A temperature regulated water bath of (60cm x 40cm x 16cm) and 1.2 kW power was equipped with a stainless steel electrical stirrer (1.2 kW) and coupled with voltage variac box. The clarifier was a 3- liter Pyrex flask with a discharge valve at the bottom and a tap near the top, for the collection of treated wastewater. Air was introduced via a pre-calibrated rotameter with and an air sparger to ensure even distribution. A Pyrex burette was used for the delivery of nutrient (phosphoric acid).
Some supplementary equipment were used including an incubator (Memmert, Germany); a microscope (Olympus); an autoclave for sterilization (Express, England); a Digital Grating Spectrophotometer (Pye unicam, England); and a Universal Pocket Meter Multiline P4 including: I. pH combined electrode with integrated temperature probe Sen tix 41; and II. Dissolved oxygen (DO) probe, Cell Ox 325.
Procedure
The samples were taken from outline area from the wastewater of the vegetable and chrome tannery, in equal volumes. The samples were first screened to remove hair and skins pieces and then neutralized by adding sulphuric acid (50 wt%) to a final pH of (7-9). Sedimentation (settling) process was then carried out to reduce the solid content to about 65% within 2-3 h. A specified volume of the sample (30 Lit) was placed in the bath. The microorganism (activated sludge) where added to the water sample in the bath, and aeration was started. Following the Box-Wilson method of experimental design the operating parameters used, were in the range;
T = 20 - 45oC; t = 5-10 hour PO4 concentration = 5-20 mg/LitAfter the biochemical treatment, a polyelectrolyte at a rate of 15 mg/L was added to the mixture in the bath for a period of one hour to improve the process of sedimentation. In the natural sedimentation stage, the sample was taken from the bath and clarified for about 3 h, and then a specified volume of the clarified water was taken for the BOD5 measurement.
Methods of examination
The TSS and volatile suspended solids (VSS) tests were done according to the WHO methods for pollution control, 1982. The (DO) concentration was measured using a (Cell DX 325) type device, which consists of a gold-metal electrode (Wissen Schuftliche Tech. Werk., Germany). The accuracy of this method is (0.1 mg/Lit). The readings were checked versus titration method. The titration was carried out using standard 0.025 N sodium thiosulfate and starch as an indicator.
The dilution, the BOD5 measurement and the determination of the phosphate concentration were carried out in accordance with standard methods.25 To perform bacteriological tests, the preparation of agar plate as well as the isolation of discrete colonies from a mixed culture was carried out according to the published methods.26
This work deals with effect of three variables (temperature, aeration period and phosphate concentration) on BOD5.
Postulating the mathematical model
A second order polynomial equation was employed in the range of the independent variables. Three variables were considered. The general form of a 2nd polynomial equation can be given as follows:
Y = B10 + B11X11 + B12X12 + B13X13 + B14X213 + B15X212 + B16X213 + B17X11X12 + B18X11X13 + B19X12X13 ……..(1).
The coded and real variables as well as the real values of BOD5 are given in Table 1. The data of Table 1 were fitted to equation (1) so that the regression analysis of central composite design to the approximating model to obtain the optimum conditions of the process. The coefficients of polynomial equation were evaluated. Thus, the best form of equation 1 is:
Exp No. |
Coded Variable |
Real Variable |
BOD5 |
||||
X11 |
X12 |
X13 |
x11, ̊C |
x12, hr |
x13, mg/Lit |
||
1 |
-1 |
-1 |
-1 |
25.3 |
6 |
8.2 |
350 |
2 |
1 |
-1 |
-1 |
39.7 |
6 |
8.2 |
420 |
3 |
-1 |
1 |
-1 |
25.3 |
9 |
8.2 |
300 |
4 |
1 |
1 |
-1 |
39.7 |
9 |
8.2 |
385 |
5 |
-1 |
-1 |
1 |
25.3 |
6 |
16.8 |
330 |
6 |
1 |
-1 |
1 |
39.7 |
6 |
16.8 |
380 |
7 |
-1 |
1 |
1 |
25.3 |
9 |
16.8 |
290 |
8 |
1 |
1 |
1 |
39.7 |
9 |
16.8 |
365 |
9 |
-1.732 |
0 |
0 |
20 |
7.5 |
12.5 |
325 |
10 |
1.732 |
0 |
0 |
45 |
7.5 |
12.5 |
445 |
11 |
0 |
-1.732 |
0 |
32.5 |
5 |
12.5 |
335 |
12 |
0 |
1.732 |
0 |
32.5 |
10 |
12.5 |
268 |
13 |
0 |
0 |
-1.732 |
32.5 |
7.5 |
5 |
310 |
14 |
0 |
0 |
1.732 |
32.5 |
7.5 |
20 |
288 |
15 |
0 |
0 |
0 |
32.5 |
7.5 |
12.5 |
288 |
16 |
0 |
0 |
0 |
32.5 |
7.5 |
12.5 |
290 |
17 |
0 |
0 |
0 |
32.5 |
7.5 |
12.5 |
288 |
18 |
0 |
0 |
0 |
32.5 |
7.5 |
12.5 |
288 |
19 |
0 |
0 |
0 |
32.5 |
7.5 |
12.5 |
288 |
20 |
0 |
0 |
0 |
32.5 |
7.5 |
12.5 |
288 |
Table 1 The Coded and Real Variables of The Calculations Of BOD5 Using Central Composite Method
Y = 288.498 + 34.846X11 + 18.289X12 + 9.892X13 + 36.896X211 + 9.151X212 + 7.317X213 + 5X11X12 + 3.75X11X13 + 3.749X12X13 …..(2)
Correlation coefficient, R = 0.977,
Percentage square error (S) = 1.8%.To test the significance of each term in equation (2), the F- distribution test was used employing the variance of each term in multivariable correlation, according to (Table 1).27 The calculations indicated insignificant interaction between the variables (X1X3, X1X2, X2X3) are. Thus the best form of the relationship is
Y = 288.498+ 34.846X11+ 18.289X12+ 9.892X13+ 36.896X211+ 9.151X212 + 7.317X213 …………..…..(3).
The optimization process 28 was applied to equation (2) to find the optimum operating conditions and the results indicate the following conditions to attain the minimum BOD5 = 256 mg/L:
X11 = Temperature = 32.5 ̊C,
X12 = Aeration period = 10 h,
X13 = Phosphate concentration = 16.8 mg/L.
Effect of operation variables on BOD5
Effect of Temperature: Figure 2 shows the influence of temperature on BOD5 at different aeration periods at fixed phosphate concentration (16.8 mg/L). It is clear that, the BOD5 decreases with increasing temperature down to a value of 250 mg/Lit at 32.5 ̊C and aeration period of 10 h. Beyond this temperature, the BOD5 values increase.
Figure 3 shows the effects of temperature on BOD5 at various phosphate concentrations and constant aeration period of 10 h. The BOD5 reached a value of 250 mg.L-1 at temperature of 32.5 ̊C and phosphate concentration of 16.8 mg/L. Figure 4 shows the response surface function developed by the model considering temperature and aeration period. The response obtained is minimum BOD5 at temperature of 32.5 ̊C and aeration period of 10 h.
It is clear that increasing in temperature from 20 ̊C to 32.5 ̊C, leads to an increase in the activity of microorganisms to degrade the organic material. Beyond 32.5 ̊C, the activity of microorganism decreases.14,28,29 The trend agrees with the theory which states that the BOD5 reaction rate coefficient and the concentration of organic material in start reaction are directly affected by temperature, as given by the following equation:
BOD = L0(T)(1-10-KTt) (4)
Where, according to Van’t Hoff-Arrhenius law
KT = K20θ(T-20) ………………………………………(5)
and
L0(T) = L0(20)(1 + 0.02(T – 20)) …………………………..(6)
The constant θ, as given by Euiso28 is equal to 1.047. Equations (5) and (6), thus, indicate that the increase in temperature influence the reaction rate coefficient and the concentration of organic material at the start reaction. Hence, the increase in temperature until 32.5 Co would increase KT and Lo(T) and reduces BOD.29
Effect of aeration period
The effects of the aeration period on the BOD5 at various temperatures and phosphate concentration are shown in Figures 5 & 6, respectively. The BOD5 values decrease with the increase of aeration period and reaches 250 mg/L at a temperature of 32.5 ̊C at a fixed phosphate concentration of 16.8 mg/L (Figure 5). Figure 6 indicates that aeration period of 10 h is enough to drop the BOD5 value down to 250 mg/L at a phosphate concentration of 16.8 mg/Lit and constant temperature of 32.5 ̊C. The increase in the aeration period to a certain limit lowers both the BOD5 and the MLSS, since there will be longer time for the microorganism to decompose the organic matter into simpler materials.30
However, the increase in the oxygen supply above this limit will improve the growth and reproduction of the microorganism and needed high cost for aeration basin.29 The competition between these microorganism for nutrient, may lead to starvation and thus a reduction in the number of microorganism.31
Effect of phosphate
Figure 7 shows the influence of phosphate concentration on BOD5 at different temperatures using constant aeration period of 10 h. The BOD5 reaches a value of 250 mg/L at phosphate concentration of 16.8 mg/L and at a temperature of 32.5 ̊C. Figure 8 shows the influence of phosphate concentration on BOD5 at various aeration periods and constant temperature. The BOD5 decreases when phosphate concentration increases up to a level of 16.8 mg/L. Beyond this level, the BOD5 increases slowly since more phosphate concentration helps algae growth. When these algae die and decay they give organic material which increases the BOD5. Thus it may be assumed that, the input of 1 mg phosphorus lead to the growth of about 100 mg of algae dry matter.32 Figure 9 shows isometric relationship of the BOD5 with aeration period and phosphate concentration. The response obtained is minimum (BOD5) at aeration period 10 h and phosphate concentration of 16.8 mg/L.
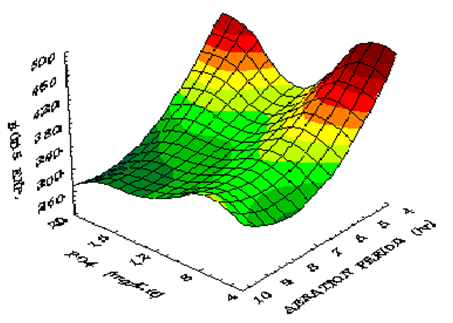
Figure 9 Response surface of the combined effects of Aeration Period and Phosphate Concentration on BOD5.
Microorganisms require certain nutrients for growth. The basic nutrients of abundance in normal raw sewage are carbon (C), nitrogen (N) and phosphate (P) with the ratio of C:N:P of approximately 100:10:1.32 Phosphorus is an essential element, it is part of the structure of DNA and RNA, and is an important intermediate in metabolism.31
Bacteriological tests indicated the presence of bacteria and protozoa in the ASP. The types of bacteria found on the surface after incubation were: I. Staphylo cocci spp. and II. Bacilli. Meanwhile, fungi did not appear on culture, since they favor pH lower than the pH of ASP.
Applications of optimum condition for wastewater treatment plant
After carrying out the preliminary processes, the wastewater samples were taken from the effluent of two tanneries, with their BOD5 values reduced from 760 mg/L to 610 mg/L. The optimum conditions that have been obtained above (Temperature = 32.5 ̊C; Aeration period = 10 h; and Phosphate concentration = 16.8 mg/L) were used for the biochemical treatment. The treatment resulted in a decrease in the BOD5 form 436 mg/Lit to 75 mg/L in laboratory. However, the actual BOD5 value of the effluent from the first aeration basin in plant was 100 mg/L.
The biochemical treatment of vegetable tannery wastewater can be best performed at the following conditions: Temperature, 32.5 ̊C; Aeration period 10 hr and Phosphate concentration, 16.8 mg/L. With such conditions, The BOD5 value could be reduced from 436 mg/L down to a value of 30 mg/L. Only bacteria and protozoa were found as microorganisms in the activated sludge, because the alkaline condition and unaeration are not suitable for the living of fungi. Waste water treatment with Reverse Osmosis technology is the best option for treating high conductivity of global waste water from tannery industry. Feasibility of reusing tannery waste water by combinations of existing conventional activated sludge process, with Ultra filtration, Nano filtration and Reverse Osmosis treatment technologies were studied.20
None.
The author declares no conflict of interest.

©2017 Barbooti, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.
 World Immunization week is celebrated in the last week of April. This highlights the importance of vaccination to all age groups to protect from diseases. So, we are enthusiastic to invite all to submit articles which help our readers in raising the awareness on this vaccination for vaccine-preventable diseases. You can also avail a best discount of 35% for your submissions in our Journal of Bacteriology & Mycology: Open access (JBMOA).
World Immunization week is celebrated in the last week of April. This highlights the importance of vaccination to all age groups to protect from diseases. So, we are enthusiastic to invite all to submit articles which help our readers in raising the awareness on this vaccination for vaccine-preventable diseases. You can also avail a best discount of 35% for your submissions in our Journal of Bacteriology & Mycology: Open access (JBMOA).