International Journal of
eISSN: 2573-2838


Research Article Volume 2 Issue 1
Department of Electrical Electronics Engineering, Universidad de los Andes, Colombia
Correspondence: Johann F Osma, CMUA, Department of Electrical and Electronics Engineering, Universidad de los Andes, Cra 1E No 19a 40, Bogota, DC 111711, Colombia
Received: October 21, 2016 | Published: January 24, 2017
Citation: Barbosa NL, Segura C, Osma JF. Electro-immuno sensors: current developments and future trends. Int J Biosen Bioelectron. 2017;2(1):20–24. DOI: 10.15406/ijbsbe.2017.02.00010
Although immunoassays have been widely used in laboratories and medical facilities for the effective detection of different proteins, their long times and costs have produced the need of developing substitute technologies. Currently, electro-immuno sensors have appeared as a solution that can be turned into point-of-care devices for diagnosis purposes. Electro-immuno sensors are biosensors that use an antibody as the recognition element and electrochemical techniques as tranducers, and have been principally developed in a disposable format. This review attempts to describe the current state-of-the-art of immune tests used in laboratories and medical facilities for the effective detection of a wide number of proteins, and how they are seemed to be migrating from chromatographic measurements to electrochemical ones. Finally, it exposes the general considerations that should be taken into account in the development of commercial electro-immuno sensors, in which concepts as the Internet of Things (IoT), cloud computing and wireless connectivity are explored.
Keywords: electro-immuno sensors, antibody-based biosensors, electrochemical transducers, hydroxyapatite powder, antibody immobilization, disposable electro-immuno sensors, redox reactions
Iot, internet of things; ELISA, enzyme-linked immuno sorbent assay; WB, western blot; IHC, immunohistochemistry; ICC, immunocytochemistry; FACS, fluorescence-activated cell sorting; IP, immuno precipitation; ELISPOT, enzyme-linked immuno spot; PSA, prostate specific antigen; IDEs, interdigitated electrodes; HPV, human papilloma virus
Biosensors have been mostly used in the food industry, medical and biological fields, among others, where physical and chemical sensors cannot accurately measure all variables. Biosensors are usually comprised by a biological recognition element and a transducer.1,2 Among the different commercial biosensors, the most common recognition elements are DNA,3 enzymes,4 aptamers5,6 and antibodies.7,8 DNA-based biosensors detect specific analytes by their cleavage and ligation to a functionalized DNA strand.9 Enzymatic biosensors immobilize an enzyme to an electrode to facilitate the transport of electrons from the enzyme to the electrode, produced by specific reactions at the active site of the enzyme.2 Aptamers are chemically synthesized oligonucleotides10 that provide high specificity and sensitivity towards a specific target. Antibody-based biosensors recognize a specific antigen from the immobilization of a monoclonal or polyclonal antibody.8 In general, transducers vary according to the end user towards which the sensor is thought. Among others, transducers include electrochemical responses, mass changes, optical absorption or transmission, and thermal readings.2
During the last decades, several immunoassay techniques have appeared as a response to the need of having specific and sensitive tests for antigen recognition. The methods by which the recognition is perform varies from one to another, but the presence of at least one recognition antibody remains as a constant. Among others, the most common immune tests used in laboratories and medical facilities are the enzyme-linked immuno sorbent assay (ELISA), western blot (WB), immunohistochemistry (IHC), immunocytochemistry (ICC), fluorescence-activated cell sorting (FACS), immuno precipitation (IP), and enzyme-linked immuno spot (ELISPOT). Figure 1 shows a general diagram of these immunoassays.
ELISA is a widely used technique for the recognition of antibodies’ antigens within a sample by means of the biochemical recognition of an enzyme.11 In this technique, specific antibodies are conjugated to an enzyme that catalyzes a colorimetric molecule. Spectrophotometric measurements are used to determine the antigen concentration in the sample. ELISA tests can be performed in a qualitative way, in which the results are positive or negative by statistical methods; or quantitative, in which a standard curve from spectrophotometric measurements can be used to quantify the concentration of the antigen.12
WB is a technique for the detection of specific proteins in a sample through the electrophoretic transfer from a separating gel to a blotting matrix.13 WB uses available monoclonal or polyclonal antibodies for the detection of specific polypeptides. This allows the optimal combination of the high resolution from electrophoresis and the high sensitivity from immunoassays.14 Antibodies are labeled so that the presence of a particular protein can be detected by means of chemiluminescence.
As performed by WB technique, IHC is a method for specific protein recognition through antibody binding. However, in IHC, protein expression can be characterized directly from the intact tissue.15 For this reason, this technique has been used in the diagnosis of tumors16 and carcinomas,17 where proteins play a decisive role. Similarly, ICC is used to study the sub cellular distribution of proteins through fluorescent labeled antibodies.18 Contrarily to IHC, ICC has shown to work during in vivo measurements of amyloid fibrils by human lysozyme.19 However, most successful ICC applications use antibodies binded to oligonucleotides for in situ measurements.20,21
FACS is a technique in which the quantification of a fluorescent signal is used to separate cells by a particular physical characteristic (e.g. cell-size, fluorescence intensity).22 This method has been primarily used in research experiments, although Breser et al.23 has shown its potential for detecting prostate inflammation in autoimmune prostatitis. IP technique enables the isolation and purification of complexed and individual proteins by immobilizing antibodies onto solid substrates.24 This technique can be used to analyze the chromatin of a particular cell, in which DNA/protein interactions are observed,25 and can be used to determine the binding sites of chromosomal proteins. This correlation technique between proteins and gene expression has contributed to the growth of bioinformatics libraries that help scientists to understand the effect of specific proteins in living organisms.
ELISPOT assay was created for the detection of antigen-specific immune response in single cells.26 Nowadays, ELISPOT is used for a variety of applications like monitoring the response of immunotherapeutic treatment in cancer patients,27–29 and the specific immune response in patients with infectious,30 neoplastic31 and autoimmune32 diseases. All the immunoassays mentioned above have been part of the usual protein quantification and detection techniques. Moreover, all of them are based in recognition by chromatographic principles, require specific technologies, and trained individuals to carry out the test. This has created difficulties in the integration of the current immunoassay techniques for point-of-care devices, where non-trained individuals should be able to carry out the tests and get reliable results.2 In response, protein recognition by electrochemical techniques has emerged and has been integrated in what is known as electro-immuno sensors.
Electro-immuno sensors are antibody-based biosensors that use electrochemical transducers. The charge transport capacity of the electrodes of these sensors can be measured by cyclic voltammetry (CV) or as a change in the electrical impedance by electrochemical impedance spectroscopy (EIS). These sensors have been recently used in a variety of point-of-care detection devices, and have shown to be promising for liver function,33 wound infection biomarkers34 and cancer biomarkers35,36 detection. This review attempts to describe the migration of electrochemical techniques such as CV and EIS from characterization to point-of-care detection techniques. Finally, it exposes the general considerations that should be taken into account in the development of commercial point-of-care devices based on electro-immuno sensors, in which complementary concepts as the Internet of Things (IoT), cloud computing and wireless connectivity are explored.
Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS)
CV is an electrochemical technique that allows the study redox reactions at the electrode solution interfaces. The procedure uses a reference, a working, and a counter electrode to measure the current that is generated in an electrochemical cell when there is an excess of voltage from that predicted by Nernst equation.37 The conditions under which the CV is performed are usually controlled with a potentiostat. A periodic potential is applied between the working and reference electrode to produce redox reactions at the working electrode. The counter electrode is used to avoid a current flow between the working and reference electrode that might change the applied voltage.38 A measurement of the current generated between the counter and working electrode in relation with the potential applied between the reference and working electrode gives information of the oxidation and reduction of the analyte.
EIS is a technique that studies an electrochemical system by observing its behavior as an idealized model circuit with discrete electrical components. EIS applies an alternating potential between two electrodes and measures the current generated through the sample. The potential is applied at different frequencies to generate the impedance spectrum of the sample. Contrary to metal samples, where the charge carriers are electrons, in biological samples, ions constitute the main charge carriers and thus, the conductivity is dependent on their concentration and diffusion coefficients.39
CV and EIS techniques have been commonly used to characterize the electrical and redox nature of different types of samples. Nevertheless, they have recently become a method for characterizing biological samples and understanding the behavior of biological systems. For instance, Chang et al.40 used fast-scan CV to monitor the presence of adenosine in patients during deep brain stimulation. CV allowed them to correlate the presence of microthalamotomy with neurochemical changes when introducing the stimulating electrode. Similarly, Yun et al.41 fabricated an EIS measurement system at the end of a hypodermic needle to differentiate between healthy and damaged tissues prior to biopsy. CV and EIS techniques have shown to be easily integrated in lab-on-a-chip devices2,42 for point-of-care applications based on electro-immuno sensors.
Electro-immuno sensors
In general terms, all different antigen recognition techniques used in laboratories and medical facilities can be used as a base for electro-immuno sensors. However, the modified electrode technique, where the electrode serves as the support material for the antibody immobilization, has been widely explored.43 During the measurement, electrochemical techniques play a fundamental role and can be easily adapted for multiplex immunoassays.44–46 This technique is illustrated in (Figure 2), where the presence of a binded antigen produces an electrochemical change.
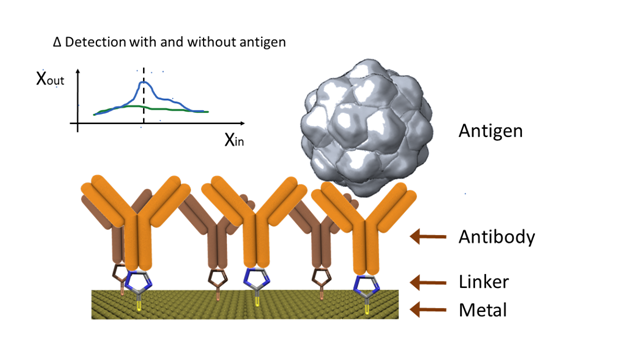
In theory, any electrode can be adapted for the immobilization of antibodies on its surface. Nevertheless, screen printed electrodes have been preferred due to their low cost, easy manufacture and suitability for mass production.47 Although there is a preference for disposable electro-immuno sensors,48–50 there is a growing trend of surface renewal for reusable purposes.51 However, the latter involves long renewal times, which increases the response time of the system.
In order to adapt the electrode for further immobilization, nano-materials such as gold nanoparticles52,53 and hydroxyapatite powder,54 membranes with ion-exchange sites,55 and polymeric films,56 are commonly used. One possible way to make the electro-immuno sensor semi-reusable is by attaching the antibodies to magnetic nanoparticles prior to their immobilization on the electrode surface. In this way, the deposition of the sensible layer can be controlled by an external magnetic field.57
Currently, there are many different manufacture methods to obtain a functional electro-immuno sensor. For instance, Duangkaew et al.58 showed a novel method to amplify the signal of a biosensor that uses a prostate specific antigen (PSA) as the recognition element. The authors developed a sandwich based biosensor in which PSA is placed between two antibodies. One of them is attached to a substrate while the other is attached to a matrix of gold nanoparticles (AuNP). The presence of AuNP facilitated the growth of gold spikes increasing the area-to-volume ratio. This increased the output current of the sensor when tested by anodic stripping analysis.
Interdigitated electrodes (IDEs) are commonly used when performing electro-immuno sensors based on EIS. Singh et al.59 Yu et al.60 and Zhang et al.61 developed electro-immuno sensors with IDEs by using rectangular arrays for measuring the presence of Ricin. Authors measured the impedance variation by CV and EIS on the IDEs. Hsieh et al.62 assembled a biosensor with ring-shaped IDEs to promote an uniform distribution and an efficient immobilization of glycated hemoglobin (HbA1c).
In the same way, Osma et al.46 designed a fabrication process of an electro-immuno sensor based on the immobilization of a monoclonal Antibody (mAb) 5051 on gold IDEs for the detection of the Human Papilloma Virus (HPV) 16. In this case, mAb 5051 was attached to a gold layer through thiols. CV measurements allowed the effective detection of HPV 16. A similar process was developed by Monerris et al.,63 where an electro-immuno sensor based on CV measurements was used to sense the presence of estrone hormone in water samples. This was attained by immobilizing a sheep anti estrone mAb on a layer of AuNPs.
Hou et al.64 and Freitas et al.,65 fabricated electro-immuno sensors that measured through EIS and CV for the detection of microcystin-LR and Cry1Ab, respectively. Similarly, Moschou et al.66 used double layers printed circuit boards (PCBs) to fabricate electrodes, reducing manufacturing time and costs. On the copper electrodes, gold and silver layers were coated for the further antibody immobilization. Measurements were performed through CV techniques. The authors compared the electro-immuno sensor with an ELISA immunoassay, showing that it was possible to detect TMB/H2O2 through electrochemical methods.
Future trends
Electro-immuno sensors have appeared as a novel trending solution for specific and high sensitive biosensors. It has been proven that using immune detection results in a feasible solution for specific protein detection in low concentrated and low volume samples. This constitutes a promising technique for point-of-care devices, in which a low sample and reagent volume requirements, easy integration and rapid response are desired.2 However, current electro-immuno sensors are still an emerging trend, and so, several aspects should be considered in order to achieve a commercial and user friendly product.
For instance, current electro-immuno sensors should consider their communication with front-end technology such as smart phones and computers, so that there is an easy monitoring and data storage. User-friendly applications must be developed to receive and process information from the sensor device. Similarly, in order to migrate electro-immuno sensors from laboratory to industrial products, their manufacture process needs to be scalable and easy to automatize. Either if they are used as point-of-care devices or as laboratory instrumentation, they should contain an user-friendly interface, which is usually approached with a sample-to-answer format.67 In addition, independent rechargeable energy sources should be considered if the electro-immuno sensor is meant to be portable. Finally, concepts as the IoT and cloud computing should be integrated with the sensors circuitry.
None.
The author declares no conflict of interest.

©2017 Barbosa, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.