Advances in
eISSN: 2377-4290


Research Article Volume 6 Issue 3
Department of ophthalmology Cairo University Egypt
Correspondence: Iman M Eissa Assistant professor of ophthalmology Faculty of Medicine Cairo University Almanial Cairo Governorate Egypt, Tel +201006383248
Received: February 18, 2017 | Published: March 8, 2017
Citation: Attya AAM, Allam RSHM, Eissa IM, Eyada O, Taha AM (2017) Correlation between Spectral Domain Optical Coherence Tomography and Microperimetric Findings before and after Intravitreal Bevacizumab Injection in Wet Age Related Macular Degeneration. Adv Ophthalmol Vis Syst 6(3): 00182. DOI: 10.15406/aovs.2017.06.00182
Purpose: To evaluate Microperimetry and Optical Coherence Tomography (OCT) in correlating macular structure/function, location/stability of fixation and preferred retinal loci (PRL) before and after bevacizumab injection in wet Age Related Macular degeneration (AMD).
Methods: 30eyes with wet AMD underwent OCT and microperimetry before receiving bevacizumab and one month after treatment. Topography/Microperimetry overlay was used to assess macular thickness/sensitivity and Location/stability.
Results: There was statistically significant improvement in macular thickness and best corrected visual acuity (BCVA) but improvement in macular sensitivity was not significant. There was significant positive correlation between baseline visual acuity (VA) ≤ 3/60 and change in VA. No significant correlation between improvement in macular thickness and change in macular sensitivity. No significant correlation between improvement in VA and improvement in macular sensitivity.
Conclusion: Microperimetry is an important tool in evaluating macular structure and function, location/stability of fixation and PRL in wet AMD.
Keywords: age related macular degeneration, microperimetry, optical coherence tomography, retinal sensitivity, bevacizumab, macular thickness
AMD, age related macular degeneration; CNV, choroidal neovascular membrane; IS/OS, inner segment/outer segment; OCT, optical coherence tomography; PRL, preferred retinal loci; RPE, retinal pigment epithelium; SD-OCT, spectral domain optical coherence tomography; VEGF, vascular endothelial growth factor
Age related macular degeneration (AMD), especially the neovascular (wet) type, accounts for most cases of severe visual loss above the age of fifty in developed world. With the introduction of intravitreal antiangiogenic agents like ranibizumab and bevacizumab, which inhibit the vascular endothelial growth factor (VEGF), major advance in treatment outcomes of neovascular AMD was achieved.1 In line with these treatment options, optical coherence tomography (OCT) has become an essential adjunct in the diagnosis and follow up of AMD cases.1 Most anti VEGF treatment regimens comprise a monthly injection for three consecutive months, followed by a maintenance phase during which injections may be repeated and follow up is usually done by assessing the visual acuity, fundus and OCT.2 While OCT can give an idea about the macular thickness and morphology, as well as the amount of exudation, it does not give any idea about the retinal function. There is clinical evidence that some patients with stable visual acuity and central retinal thickness on follow up may still have deteriorating retinal sensitivity. This is usually due to subclinical choroidal neovascular membrane (CNV) activity.3
Microperimetry assesses central retinal light sensitivity over certain points, and topographically correlates this sensitivity to the anatomical thickness. Several previous reports have highlighted the benefit of this structure-function correlation in cases of AMD.4 Microperimetry allows for automated assessment of macular function as well as automated correction for eye movements, as unstable fixation.4,5 It also gives an idea about the fixation pattern and the preferred retinal loci (PRL) of patients. These PRL are mapped in relation to fixed anatomical landmarks, so they can be re-examined in subsequent examinations, a point which allows for future comparisons.5 Incorporating microperimetry in the follow up of neovascular AMD, after anti VEGF injection could thus be a beneficial tool to evaluate retinal sensitivity even in cases with stable visual acuity and stable macular morphology on OCT. A previous study showed significant increase in mean retinal sensitivity with a decrease in absolute scotoma size 6months after bevacizumab injection,6 a point which further encouraged us to use microperimetry in parallel with OCT in following up patients with neovascular AMD. While most studies only correlated macular structure and function by using OCT and microperimetry,7 our study aimed at correlating these two parameters as well as comparing them before and after anti VEGF injection.
Our study is a non-randomized prospective, interventional study that was carried out between July 2013 and August 2015 at Kasr El-Ainy university hospital and LASER and ophthalmology research centre. Data collection conformed to all local laws and was compliant with principles of the Declaration of Helsinki. The study was approved by ethical committee of ophthalmology department, Cairo University.
Study subjects
The study included 35eyes (27patients) having wet AMD (classic, atypical or occult) scheduled for intravitreal bevacizumab treatment (at month 0, 1, 2 and 3). Only eyes who did not receive previous intravitreal injections were included in the study. We excluded eyes with AMD that received previous intravitreal injections, eyes with by vitreous hemorrhage or massive exudation leading to exudative retinal detachment, history of diabetes mellitus or a systemic disease affecting the eye, history of previous intra-ocular surgery except uneventful cataract surgery as well as eyes having media opacity interfering with OCT imaging or microperimetry.
Patients’ evaluation
Clinical assessment: History was taken to identify the presence of any known risk factors for CNV including age at time of presentation, sex, occupations with prolonged exposure to sunlight and tobacco smoking Examination was done prior to each intravitreal injection and after four months (one month following the third injection). All eyes underwent routine ophthalmologic examination in addition to evaluation of AMD in the form of Amsler grid testing (chart no.1).
Structural assessment: Spectral domain optical coherence tomography (SD-OCT) using OPKO Spectral OCT/SLO combination imaging system; OPKO Instrumentation, LLC, USA, version 1.89. Central foveal thickness (CFT) was measured in microns, inner segment/outer segment (IS/OS) junction integrity and Retinal Pigment Epithelium (RPE) band were assessed. The IS/OS was graded as complete if the hyper reflective line (Interface) between the IS/OS was present, as discontinuous if the line was interrupted and the segments were only partly visible, or as completely absent.7 The RPE layer was graded as complete if the hyper reflective band was continuous, as discontinuous if the RPE was disrupted and only partly visible, or as absent if no band was visible.7
Functional assessment: Macular Microperimetry was done using OPKO Spectral OCT/SLO combination imaging system; OPKO Instrumentation, LLC, USA version 1.89. The following program was used: Pattern: Square 5 x5–90; Target size: Goldmann III yet in cases where it was not seen by the patient, Goldmann IV was used; Strategy: 4-2. Topography and Microperimetry overlay was used, comparison between the microperimetry findings (quantitative) and Amsler grid (qualitative) during follow up was carried out.
Surgical technique
Before injection, topical proparacaine hydrochloride was applied to the ocular surface followed by eye sterilization, sterile draping preparation with 5% povidone iodine. A cotton-tipped applicator soaked in proparacaine hydrochloride was then applied to the injection site 4 mm posterior to the limbus in phakic patients (3.5 mm in the pseudophakic patients). Commercially available bevacizumab (Genentech Inc., San Francisco, CA, USA) (100 mg vial of 25mg/ml concentration) (0.1 ml of 25 mg⁄ml) was prepared for each patient and was placed in a tuberculin syringe by a physician using aseptic techniques. A paracentesis was done in all cases to avoid IOP elevation. Patients were instructed to use topical ciprofloxacin q.i.d for 5days and were examined 1 week after injection. The procedure was done once monthly for three successive months.6
Statistical method
Data analysis was performed using Statistical Analysis Systems (SPSS ver.16). Numerical data was summarized using means and standard deviations or median & ranges. Categorical data was summarized as percentages. Comparisons between baseline and follow up data were done by McNemar test and Wilcoxon Signed Ranks test. Mann-Whitney Test was used for nonparametric data comparison. The chi-square test was used to compare between the groups with respect to categorical data. The Spearman correlation for nonparametric data was used to assess the degree of association between the numeric variables. P-values <0.05 were considered significant. For statistical reasons, point thickness and point sensitivity values were summarized into five values using the mean value of 4 points at every quadrant and the central point, which differs from the point of fixation.
35eyes (27patients) were enrolled in this study. 5eyes were excluded for not completing their follow up, so 30eyes (23patients) were included in statistical analysis. OCT scan and microperimetry were done at baseline and follow up one month after the end of bevacizumab loading dose (once monthly for three successive months).
Clinical assessment
The mean age of the study group was 65.2±1.15years (from 55 to 76years). The study included 8males and 15females with male to female ratio (1: 1.87). Examination was done prior to each intravitreal injection and after four months. Clinical and anterior segment examination data at baseline was compared to the last follow-up and is summarized in (Table 1). The change occurring in VA ranged from -0.17 to 0.6 with median value 2/60 (0.03). There was an insignificant weak negative correlation between baseline VA and the change occurring in VA after treatment (r=-0.17, p=0.98). Further statistical analysis revealed that baseline VA of 3/60 (0.05) or less improved significantly compared to follow up (p=0.001) and had a statistically significant moderate positive correlation to the change occurred in VA (r=0.39, p=0.03), while baseline VA of more than 0.05 had an insignificant improvement (p=0.07) and an insignificant correlation to change in VA.
|
|
Baseline |
|
Follow-up |
P-Value |
|
Median |
Range |
Median |
Range |
|
VA* |
3/60(0.05) |
1/60(0.016)–6/12(0.5) |
6/36(0.13) |
1/60(0.016)-6/9(0.7) |
<0.001 |
IOP† |
16 |
20-Oct |
14 |
20-Dec |
0.18 |
(mmHg) |
mean 15.8±2.7mmHg |
14.67±2.55mmHg |
|||
Lens Status |
No significant change apart from 3eyes that developed visually non-significant anterior cortical cataract at follow up that was not present at base line |
0.62 |
|||
Spherical Equivalent in Diopters |
At baseline refractive error ranged from (+6) to (-7) with a median value (-0.5), with no significant change at follow up |
0.27 |
|||
Table 1 Clinical and anterior segment examination data at baseline compared to the last follow-up visit
*V.A, visual acuity; †IOP, intraocular pressure;
Biomicroscopic fundus examination using a +90diopters lens was done and data is summarized in (Table 2). Other biomicroscopic findings (hard or soft drusen, pigmented macular lesions or clinical CNV) did not significantly change all through follow up. Evaluation of AMD using Amsler grid test at baseline visit compared to follow up visit showed decreased In the presence of blurred lines from 9 to 3eyes (p=0.07), curved lines from 10 to 7 eyes (p=0.45), positive scotoma from 4 to 3eyes (p=1.00) and number of eyes who cannot see small squares from 6 to 3 (p=0.37) with increased presence of negative scotoma from 0 to 6 eyes (p=0.03).
|
Baseline |
Follow up |
P-value |
Macular Hemorrhage |
12 |
1 |
0.001 |
Macular Scar |
5 |
11 |
0.03 |
Submacular Hemorrhage |
6 |
2 |
0.12 |
Clinically Detectable Macular Edema |
3 |
1 |
0.62 |
Table 2 Biomicroscopic fundus examination data
Structural assessment
OCT scans were obtained at baseline and one month after the last injection. The thickness map, RPE band, IS/OS integrity and presence of intraretinal or subretinal fluid were compared at baseline and last follow up as follows:
Thickness map: Baseline CFT and average macular thickness values were compared to corresponding follow up values. The mean of all point thickness values at baseline was calculated and compared to the mean of the follow up values as illustrated in (Table 3).
|
Baseline |
Follow up |
P-value |
Percentage of change median% |
|
Median (range) |
Median (range) |
|
|
*CFT |
308 |
218 |
0.03 |
5.7 |
(98 -672) |
94-519 |
|||
Average Macular |
305.5 |
238 |
0.003 |
11.74 |
Thickness |
(172 -508) |
117-494 |
||
Total Mean Thickness of All Points |
308 |
265.64 |
0.001 |
9.02 |
(233.2 -408.7) |
(208.12-430.68) |
|||
Thickness Upper Nasal |
301.5 |
266.75 |
0.01 |
16.8 |
(171 -532) |
(161-495) |
|||
Thickness Upper Temporal |
303.12 |
257.12 |
<0.001 |
13.03 |
(222 -566) |
(174-319) |
|||
Thickness Lower Nasal |
304.5 |
280.5 |
0.15 |
6.2 |
(159 -508) |
(158-545) |
|||
Thickness Lower Temporal |
277.75 |
254.88 |
0.01 |
4.52 |
(208 -438) |
(142-390) |
|||
Thickness Of Central Point |
314.5 |
250.5 |
0.005 |
2 |
|
(72 -652) |
(117-432) |
|
|
Table 3 Statistical values of point thickness map measured in microns
*CFT, central foveal thickness
IS/OS junction: The integrity of IS/OS junction graded according to its integrity as continuous, discontinuous, completely absent or cannot be assessed as in (Figure 1).
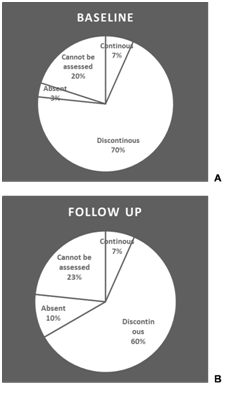
Figure 1 Pie-chart representation of the percentage of cases in each category of the IS/OS junction at base-line (A) and at follow up 1 month after last injection (B).
RPE/choriocapillaris complex layer: OCT findings in the RPE/choriocapillaris complex, CNV activity represented in the presence of subretinal or intraretinal fluid are summarized in (Table 4).
|
Baseline |
Follow up |
P value |
|
(Number of patients) |
(Number of patients) |
|
Fragmented RPE Layer |
22 |
10 |
0.008 |
Pigment Epithelial Detachment (PED) |
5 |
1 |
0.125 |
RPE Elevation |
3 |
1 |
0.5 |
Fibro Vascular Membrane/ Disciform Scar |
22 |
22 |
1 |
Drusen |
7 |
7 |
1 |
Presence of Sub Retinal Fluid |
11 |
5 |
0.03 |
Intraretinal Fluid |
30 |
10 |
0.03 |
Atrophic Cysts |
0 |
8 |
0.03 |
Table 4 OCT findings in the RPE/choriocapillaris layer
Functional assessment
Microperimetry was done using a Stimulus size (Goldmann III) for baseline examination in 18eyes as well as for follow up visit. However, Stimulus size (Goldmann IV) was used for the remaining 12eyes that could not see size III at baseline examination. The median value for false positive stimuli was 3% (zero -12%) at baseline which changed to 0.5% (zero -10%) at follow up (p=0.07). The median value for false negative stimuli was 4% (1%-10%) at baseline which changed to 5% (zero -10%) at follow up (p=0.78). On baseline visit the test was reliable in 26eyes which increased to 29eyes on follow up visit (p=0.25). However, the test duration significantly decreased from a median of 8.43minutes (3.27-43.36minutes) to 6.5minutes (2.14 -16.27minutes), p<0.001.
Point sensitivity: The mean of all point sensitivity values at baseline was calculated and compared to the mean of the follow up (Table 5). There was no significant correlation between the percentages of change in point thickness and percentages of change in point sensitivity except for the lower nasal quadrant which showed a moderate negative linear correlation (r=-0.43)(p=0.02) (Figure 2A). There was an insignificant weak negative linear correlation between baseline VA and the percentage of change in mean point sensitivity (Figure 2B).
Baseline |
Follow-up |
P value |
Percentage of change median% |
|
Median (range) |
Median (range) |
|||
Total Mean Sensitivity of All Points |
6.08 |
7 |
0.76 |
0 |
(.00 to 11.92 ) |
(.00 to 11.92 ) |
|||
Upper Nasal Sensitivity |
7.25 |
5.5 |
0.35 |
-100 |
(0 to 12) |
(.00 to 13.50) |
|||
Upper Temporal Sensitivity |
6 |
8 |
0.4 |
-4.17 |
(.00 to 13.50) |
(.00 to 17.50) |
|||
Lower Nasal Sensitivity |
7.25 |
6 |
0.89 |
-10.79 |
(.00 to 12.50) |
(.00 to 13.00) |
|||
Lower Temporal Sensitivity |
6.25 |
7 |
0.3 |
-2.27 |
(.00 to 14.00) |
(.00 to 17.00) |
|||
Sensitivity of Central Point |
2 |
2 |
0.4 |
-6.27 |
(.00 to 14.00) |
(.00 to 14.00) |
|||
Table 5 Statistical values of point sensitivity
Moreover there was an insignificant weak negative linear correlation between percentage of change in VA and the percentage of change in mean point sensitivity (Figure 2C). There was insignificant weak negative correlation between baseline IS/OS junction continuity and the percentage of change in mean sensitivity of all points (p=0.76, r=-0.06). There was insignificant comparison between the RPE layer integrity at baseline and the percentage of change in mean sensitivity of all points. The RPE layer integrity was categorized into continuous fragmented or absent RPE layer with p-values (0.4, 0.7 and 0.5 respectively).
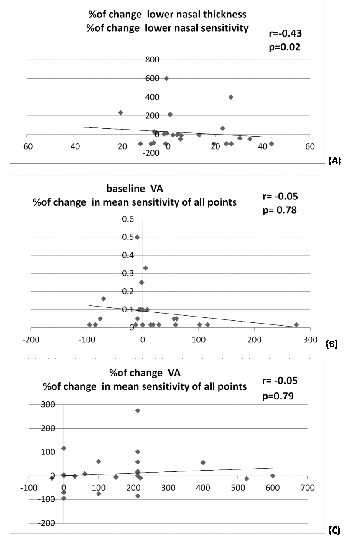
Figure 2 Line graph showing linear correlations between percentage of change in point thickness and percentage of change in point sensitivity of lower nasal quadrant (A),baseline visual acuity and percentage of change in mean point sensitivity (B), percentage of change in visual acuity and the percentage of change in mean point sensitivity (C).
The current study tackled the value of microperimetry in the assessment of the degree of macular functional changes in correlation to structural changes after intravitreal bevacizumab injection in cases of wet AMD. While VA measures the minimal angle of spatial resolution, microperimetry measures the differential light threshold, which is an indicator of retinal sensitivity and photoreceptor integrity.6,8 This gives an idea about changes affecting the retina and compromised central field in these patients. It also allows for quantification of visual sensitivity at a given retinal point, with test-retest reliability for the same point.9
In our study, BCVA significantly improved from a median value of 3/60 (0.05) at baseline to 6/36 (0.13) at the end of follow up (p<0.001) and the median value of change was 2/60 (0.03). Further analysis revealed that baseline VA of 0.05 or less improved significantly compared to follow up (p=0.001) and had a statistically significant moderate positive correlation to the change in VA (r=0.39, p=0.03), while baseline VA of more than 0.05 had an insignificant improvement (p=0.07) and had an insignificant correlation to change in VA. The improvement we found in VA agreed with other studies by Ozdemir et al.6 and Bloz et al.10 who recorded improvement in baseline BCVA.
We owe the higher improvement in BCVA in cases with initially lower vision to resolving intra-retinal edema after bevacizumab injection and absence of severe neurosensory destruction as the IS/OS junction line was intact in 40% of cases at the end of follow up. Our baseline biomicroscopic fundus findings did not show any change at the end of follow up period regarding the number of hard/soft drusen, clinically detectable CNV and pigmented macular lesions. However, a significant decrease in macular hemorrhage (p=0.001) and a significant increase in clinically detectable macular scarring (p=0.03) have occurred. To our knowledge, no previous studies have mentioned similar findings. Our study also reported a tendency to decreased reporting of blurred lines by patients on Amsler grid testing (p=0.07) which agrees with the improvement in VA. On the other hand reporting negative scotoma by patients significantly increased (p=0.03) which can be explained by the biomicroscopic finding of increased macular scarring. This parameter was also not compared in previous studies as far as the authors know.
As for baseline CFT, average macular thickness and mean of all point thickness, these values have decreased significantly at follow up (p=0.026, p=0.003, p=0.001) respectively. The decrease in thickness was significant in all three quadrants as well as the central point except for the lower nasal quadrant in which the decrease in thickness was insignificant, a point which needs further investigation. This decrease in overall retinal thickness map agrees with that reported by Bloz et al.10 in which the CFT at baseline was 324±94 microns and decreased by 109±98microns after the third injection (p<0.0001). We did not find a statistically significant difference in IS/OS line integrity at follow up. However, we found significant improvement as regards RPE fragmentation (p=0.008) as well as disappearance of intraretinal fluid present in 100% of cases before injection and decreased to 10% of cases at follow up (p=0.034). A similar significant decrease was also found as regards subretinal fluid (p=0.03). We believe that this significant decrease in both intraretinal and subretinal fluid is the main reason behind improvement in VA and retinal thickness at follow up. Apart from retinal thickness map, these other parameters (IS/OS, RPE fragmentation and intraretinal and subretinal fluid) were not specifically addressed in previous studies. Whereas some other studies reported an improvement in reading acuity, glare disability as well as contrast sensitivity after intravitreal ranibizumab.11
We further analyzed microperimetry changes and we found that the test duration has significantly decreased at follow up (p=0.001). This can be explained by the significant improvement of VA and improvement in stability of fixation, as well as the learning curve of patients. As opposed to Sulzbacher et al.7 who reported a strong negative correlation between central retinal thickness and central retinal sensitivity (r=-0.509, p=0.004), we found no statistically significant correlation between the percentage of change in point thickness and the percentage of change in point sensitivity except for the lower nasal quadrant (r=-0.426, p=0.019). This can be partially explained by the fact that the increase in retinal sensitivity in our study was not as marked as the decrease in retinal thickness or the increase in BCVA, which were both significantly high. Hartmann et al.12 had concluded in previous work that retinal sensitivity at baseline had a significant predictive value for the change in retinal sensitivity after intravitreal bevacizumab (p=0.03).
There were some limitations to our study. The rather low social and educational level of our patients who didn’t really need sharp vision in their daily life, may have led to the fact that most patients presented at a rather late stage where retinal structure and consequently sensitivity has been affected. In addition, our university hospital is a tertiary care centre receiving a lot of complicated and resistant cases. Our follow up period was also relatively short. Further studies with extended follow up periods will be beneficial.
Our study concludes that early diagnosis and proper treatment with intravitreal anti VEGF are essential for better outcomes. Not only as regards restoration of retinal structure and anatomical improvement, but also for restoration of visual function and retinal sensitivity thus giving a chance for wet AMD patients to be saved from being victims of poor vision.
None.
The authors declare that there are no conflicts of interest.
None.

©2017 Attya, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.